-
Platform
Back
DigiCert ONE Integrations
-
Solutions
Back
-
Buy
Back
Signing Certificates
Everything you need to secure your site.
- Company
-
Resources
Back
-
Support
Back
Resources
Contact Support- Americas
- 1.866.893.6565 (Toll-Free U.S. and Canada)
- 1.801.770.1701 (Sales)
- 1.801.701.9601 (Spanish)
- 1.800.579.2848 (Enterprise only)
- 1.801.769.0749 (Enterprise only)
- Europe, Middle East Africa
- +44.203.788.7741
- Asia Pacific, Japan
- 61.3.9674.5500
- Americas
-
Language
- Contact us
The Medical Device Manufacturing Toolkit
Your resource for navigating the complex
world of regulations, compliance, and total
security across the medical device lifecycle.
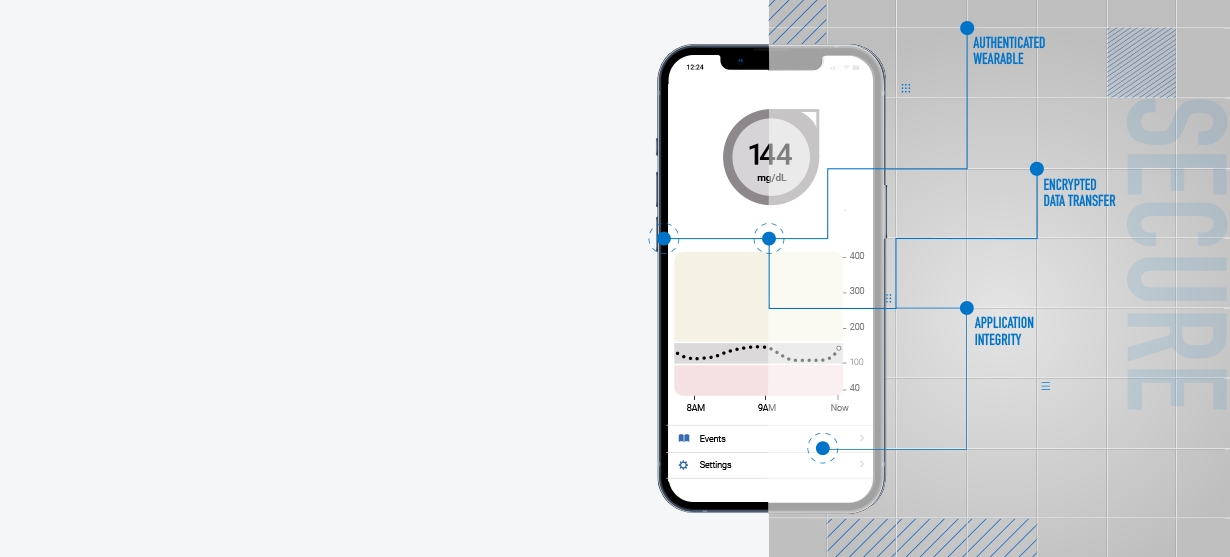
DIGICERT IoT TRUST MANAGER
Case Study: B. Braun
Patients in more than 160 countries count on connected medical devices built by B. Braun. And B. Braun counts on DigiCert for digital trust.
53%
of connected and IoT devices in hospitals have known vulnerabilities
6.2
vulnerabilities per medical device
40%
of end-of-life medical devices lack security patches or upgrades
Learn More

CHECKLIST
The 7 Habits of Trustworthy Medical Devices

SOLUTION BRIEF
Digital Trust in Connected Healthcare

EBOOK
Device Trust: Securing the Future of Smart Technology

BLOG
Healthcare Data and Quantum Computing

BLOG
Securing Diabetes Monitors

BLOG
Securing Next Gen 9-1-1

Connected Healthcare Threats can Have Catastrophic Consequences
The rise in in-home medical device use offers patients more medical flexibility than ever before. But more connections mean more attack vectors, posing a massive threat to patients and the manufacturers and providers the public entrusts with their lives.
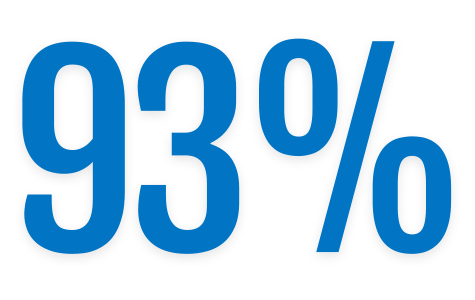
2024 State of Digital Trust Report
of the device manufacturers surveyed have experienced
- Data breaches
- Outages
- Brownouts
Learn How DigiCert Solutions Can Help You Achieve Total Security and Compliance Across the Medical Device Lifecycle
-
Company
-
My Account
-
Resources
-
Sites
-
© 2026 DigiCert, Inc. All rights reserved.
Legal Repository Audits & Certifications Terms of Use Privacy Center Accessibility Cookie Settings

